Stranded seeds in braided monofilament suture
The IsoStrand® seed chain consists of 10 IsoSeeds with standard seed-spacer configuration and a 1.0 cm center-to-center spacing. It can accomodate either IsoSeed® I25.S06 or IsoSeed® I25.S17plus.

Key Features
Good visibility
IsoStrand® is available with either I25.S06 or I25.S17plus source model designs. Both seed types are visible under X-ray, fluoroscopy, CT and MR and additionally show good ultrasound visibility.
Low migration rates
One advantage of strands is their low migration rate. IsoStrand® is specially designed to reduce the migration rates still further. IsoStrand® consists of seeds and hourglass-shaped spacers which are embedded in a braided monofilament suture. This special design enables reliable fixation within prostate tissue and helps to reduce seed migration.
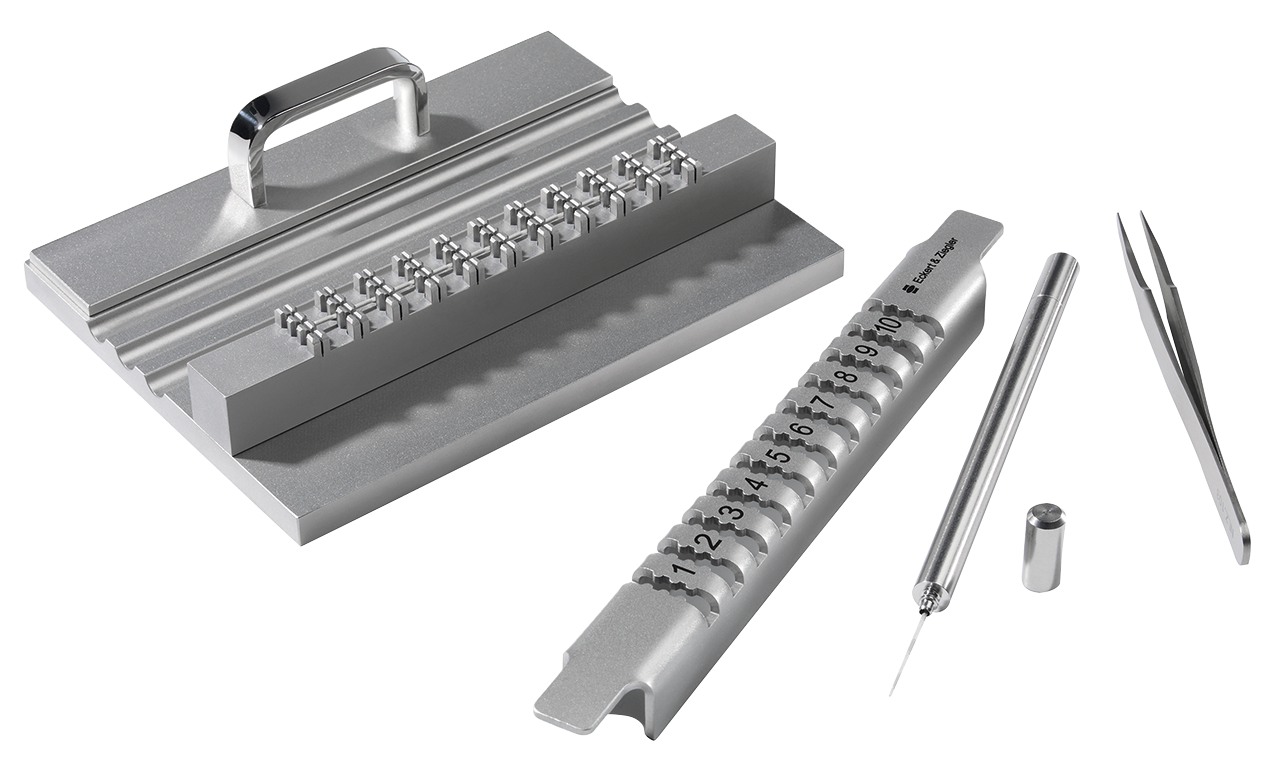
IsoStrand® accessories
Accessories include:
Short delivery times
Our production processes and intelligent stock management, with facilities centrally located in Europe, ensure fast and reliable delivery times. IsoStrand® can generally be delivered, allowing flexible patient scheduling.
Measurement
The activity of IsoStrand® can very quickly and easily be measured. This is of particular importance for clinics assaying 100% of seeds.
Calibration to NIST and/or PTB
The certified source strength of IsoSeed® is based on air kerma measurements. The source strength of IsoSeed® I25.S17plus and IsoSeed® I25.S06 are calibrated traceable to the primary standard of the NIST (National Institute of Standards and Technology), USA and/or of the National Metrology Institute of Germany (PTB).
Guaranteed biocompatibility
The titanium capsule of IsoSeed® is made of implant grade material. The finished products have been tested for biocompatibility
Quality “Made in Germany”
The production of IsoStrand® is performed at the manufacturing facility in Berlin, Germany, operating under strict quality and regulatory standards. Eckert & Ziegler BEBIG is certified according to ISO 13485.
Patient Information Leaflets (PIL)
Patient Information Leaflets (PIL) in different languages can be found here.
Recent Publications
Product specific questions?
Eckert & Ziegler
BEBIG GmbH
Robert-Rössle-Str. 10
13125 Berlin
Germany